Related Content
Medical Cannabis — Continuing Controversy
Studies show both long-term and short-term benefits and harms associated with cannabis use, all of which need to be considered in assessing its potential medical value. In addition, prescribers may and should prescribe or "recommend" cannabis use only within the legal context allowed by respective state laws. This article summarizes cannabis pharmacology and its potential health effects as noted in the literature, and should not be construed as an opinion in support of or against the legalization of marijuana for medical and/or recreational use.
Cannabis is a broad term used to describe the various products and chemical compounds (eg, marijuana, cannabinoids) derived from different species of the cannabis plant.1 The use of cannabis has been held simultaneously in high and low esteem at various times throughout recorded history.2 Unsurprisingly, the debate over the benefits and harms of cannabis use continues today with controversy often deeply rooted in individuals' own philosophical, societal, legal, and scientific beliefs. With more than one-half of America's states having implemented policy allowing the use of legalized medical marijuana,2 health care professionals and patients alike are increasingly in need of nonbiased research based on the most current scientific evidence available.
Cannabinoids
There are more than 400 natural components found within the Cannabis sativa plant, with at least 113 being classified as "cannabinoids."3
Two kinds of cannabinoid receptors have been identified and are termed CB1 and CB2. In 1992, anandamide, which is a naturally occurring substance produced in the brain, was identified and found to interact with CB1 receptors. Since then, other naturally occurring substances that bind to CB1 receptors have been discovered, and these, together with the receptors, are termed the "endogenous cannabinoid system."4
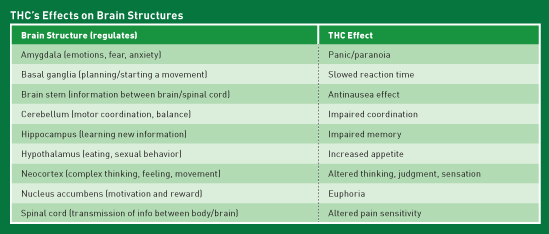
THC Effects
The cannabinoid Δ9-Tetrahydrocannabinol (THC) is responsible for the psychoactive effects of cannabis. The effects of THC are reflective of the areas of the brain with which it interacts.1 (See table.) For example, THC interacts with CB1 in the hypothalamus, leading to increased appetite, while THC interaction at the nucleus accumbens leads to the euphoria or "high" commonly associated with cannabis use.
Cannabidiol Effects
Cannabidiol (CBD) displays very low affinity to CB1 and CB2 and is nonpsychoactive, as it lacks the cannabislike intoxicating properties of THC.
CBD acts on serotonin 5-HT1A receptors and transient receptor potential vanilloid type 1 receptors, in addition to possible neuroprotective benefits resulting from antioxidant and anti-inflammatory properties. CBD may have a potential therapeutic role in the treatment of pain and inflammation and for the relief of a number of neurological disorders such as epilepsy and seizures, psychosis, anxiety, movement disorders (eg, Huntington's disease and amyotrophic lateral sclerosis), and multiple sclerosis.1
Factors Influencing THC Effects1
The amount or dose of THC in cannabis varies by plant and also between other available products, such as those that may be smoked, consumed as edibles, or applied topically. Higher doses of THC result in longer and greater physical and mental effects. Also, the effect seen with higher doses of cannabis on an individual will likely be much different from the effect of a lower dose. Furthermore, with increased use, "desensitization" is believed to occur, resulting in the need for higher doses to achieve the effects of euphoria or "high."
The method of use also influences the dosage and time before the drug effect is felt. Smoking and inhalation cause rapid but less efficient delivery of the dose; variable quantity of the drug is destroyed during burning or escapes into the air and does not reach the lungs. Oral ingestion produces different effects, according to the system in which the drug is dispersed. Generally, oral ingestion diminishes the drug effect but prolongs it.
The ratio of THC to CBD also plays a significant role in the effects seen from different products. Of interest is that this ratio may impact product selection for the treatment of a specific medical condition.
For example, CBD may have antianxiety effects that lessen the negative psychoactive effects of THC. As a result, the use of a cannabis product with higher-than-expected THC (dose or ratio) may cause the user to experience greater psychoactive effects and increased potential for toxicity, which results in a hyperemesis syndrome. On the other hand, if a plant has a greater percentage of CBD, then it essentially lowers the potency or reduces the intensity of THC's effects. Whereas one product with a higher percentage of CBD may help with anxiety, another with a lower percentage may actually result in anxiety.
Cannabinoid-Based Medications1
The FDA has licensed three drugs based on cannabinoids. Dronabinol (Marinol), which is Δ9-THC and also nabilone (Cesamet), a synthetic analog of Δ9-THC, are both approved for chemotherapy-associated nausea and vomiting and to stimulate appetite in AIDS-related wasting syndrome. A liquid form of dronabinol (Syndros) was approved by the Drug Enforcement Administration in July 2016 for chemotherapy-induced nausea and vomiting (unresponsive to conventional antiemetics) and for anorexia associated with weight loss in patients with AIDS.
The FDA has examined two additional cannabinoid-based drugs. Nabiximols (Sativex) is a cannabis extract composed of Δ9-THC and CBD formulated in a 1:1 ratio. Nabiximols is administered as an oromucosal spray for the symptomatic relief of multiple sclerosis and for use as an adjunctive analgesic treatment in cancer patients. As of September 2016, nabiximols has been launched in numerous countries, but not in the United States. In 2013, in response to the need for new treatments for intractable epilepsy, the FDA allowed investigational new drug studies of Epidiolex, a concentrated CBD oil (>98% CBD), as an antiseizure medication for Dravet and Lennox-Gastaut syndromes.
Current State of Evidence1
In one of the most comprehensive studies of recent research on the health effects of recreational and therapeutic cannabis use, a new report from the National Academies of Sciences, Engineering, and Medicine offers a rigorous review of relevant scientific research published since 1999. This report summarizes the current state of evidence regarding what is known about the health impacts of cannabis and cannabis-derived products, including effects related to therapeutic uses of cannabis and potential health risks related to certain cancers, diseases, mental health disorders, and injuries. Areas in need of additional research and current barriers to conducting cannabis research are also covered in this comprehensive report.
Substantial evidence supports cannabis as treatment for chronic pain in adults (cannabis); chemotherapy-induced nausea/vomiting (oral cannabinoids); and multiple sclerosis spasticity symptoms (oral cannabinoids).
Moderate evidence supports cannabis use as treatment for improving short-term sleep outcomes in individuals with sleep disturbance associated with obstructive sleep apnea syndrome, fibromyalgia, chronic pain, and multiple sclerosis (cannabinoids, primarily nabiximols).
However, research yields substantial evidence of statistical association between increased risk of motor vehicle crashes and cannabis use; long-term cannabis smoking and worsening respiratory symptoms, resulting in more frequent chronic bronchitis episodes; progression to developing problem cannabis use (PCU) and increasing cannabis use frequency; the severity of PCU and being male (however, the recurrence of PCU does not differ between males and females); being male and smoking cigarettes as a risk factor for progression to PCU; and initiating cannabis use at an earlier age with an increased risk for progression to PCU. Stimulant use to treat ADHD during adolescence is not a risk factor for progression to PCU.
Additional associations have been found between cannabis use and the development of schizophrenia or other psychoses, with the highest risk among the most frequent users. Also noted is the association between maternal cannabis smoking and lower birth weight of offspring.
Moderate evidence favors a positive statistical association between improved airway dynamics with acute cannabis smoking, but not with chronic use; improved forced vital capacity with cannabis smoking; and better cognitive performance among individuals with psychotic disorders and a history of cannabis use.
Additionally, moderate evidence is available on the need for cessation of cannabis smoking to improve respiratory symptoms. Negative aspects also include an increase in pediatric overdose injuries, including respiratory distress in states where cannabis is legal; an increase in symptoms of mania and hypomania in individuals with bipolar disorders (regular cannabis use); a small increased risk for the development of depressive disorders (cannabis use); an increased incidence of suicide completion (cannabis use); increased incidence of social anxiety disorder (regular cannabis use); and impairment in the cognitive domains of learning, memory, and attention (acute use).
Other negative aspects for which moderate evidence exists are a persistence of PCU and a history of psychiatric treatment, PCU and increased severity of posttraumatic stress disorder symptoms, initiating cannabis use at an earlier age is a risk factor for the development of PCU, and the development of substance dependence and/or substance abuse disorder for substances including alcohol, tobacco, and other illicit drugs.
Moderate evidence indicates no statistical association between cannabis use and worsening of negative symptoms of schizophrenia (eg, blunted affect) among individuals with psychotic disorders, or incidence of lung cancer (cannabis smoking), or incidence of head and neck cancers.
Moderate evidence exists showing that anxiety, personality disorders, and bipolar disorders are not risk factors for the development of PCU; adolescent ADHD is not a risk factor for the development of PCU; and neither alcohol nor nicotine dependence alone is a risk factor for the progression from cannabis use to PCU.
However, moderate evidence of negative findings related to cannabis use indicates that major depressive disorder is a risk factor for the development of PCU; being male is a risk factor for the development of PCU; combined use of abused drugs is a risk factor for the development of PCU; and during adolescence the frequency of cannabis use, oppositional behaviors, a younger age of first alcohol use, nicotine use, parental substance use, poor school performance, antisocial behaviors, and childhood sexual abuse are risk factors for the development of PCU.
Additional summary conclusions are available within the report including findings with little or no supporting evidence. Many of these findings are based on the lack of sufficient quality studies and may be substantiated in the future.
Challenges and Barriers
Conducting cannabis and cannabinoid research can be hampered by the following:
- regulatory barriers, including the classification of cannabis as a Schedule I substance, that impede the advancement of research;
- difficulty for researchers in gaining access to the quantity, quality, and type of cannabis product necessary to address specific research questions;
- need for a diverse network of funders to support research; and
- need for improvement and standardization of research methods (for both controlled trials and observational trials).
— Mark D. Coggins, PharmD, BCGP, FASCP, is vice president of pharmacy services and medication management for Diversicare, which operates skilled nursing centers in 10 states. He was nationally recognized by the Commission for Certification in Geriatric Pharmacy with the 2010 Excellence in Geriatric Pharmacy Practice Award.
References
1. National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press; 2017.
2. State medical marijuana laws. National Conference of State Legislatures website. http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx. Updated August 2, 2017. Accessed August 5, 2017.
3. Aizpurua-Olaizola O, Soydaner U, Öztürk E, et al. Evolution of the cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemotypes. J Nat Prod. 2016;79(2):324-331.
4. Goodman N. An overview of the endogenous cannabinoid system: components and possible roles of this recently discovered regulatory system. Erowid website, http://www.erowid.org/plants/cannabis/cannabis_pharmacology2.shtml. Updated April 17, 2011.
